A1 Anatase Titanium Dioxide,Titanium Dioxide Pigment,Nanoscale Titanium Dioxide,White Titanium Dioxide Wuxi Qijun New Material Co., LTD , https://www.tio2supplier.com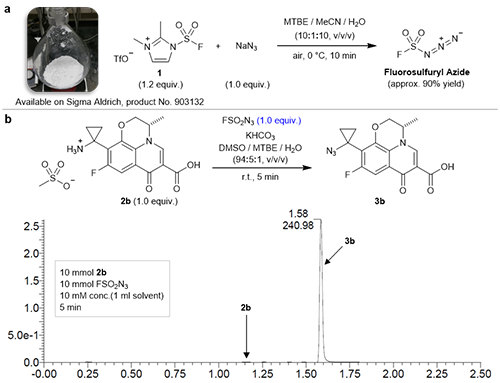
Shanghai Organic Institute discovered a new high-throughput organic synthesis method
[ Instrument Network Instrument Development ] Dong Jiajia, researcher of the Key Laboratory of Organic Fluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, found a safe and efficient synthesis of rare sulfur (VI) fluoride in the process of finding a new SuFEx reaction block. A method of the inorganic compound FSO2N3 (fluorosulfonyl azide), which simultaneously found that the compound has extremely high diazo transfer activity and selectivity for primary amine compounds. The results were recently published in the journal Nature. Meng Genyi, Guo Taijie, and Ma Tiancheng are the co-first authors of the article. Dong Jiajia and professor K. Barry Sharpless are the co-authors of the article. Shanghai Organic is the only communication unit of the paper.
With the increasing cross-infiltration between disciplines, more efficient synthesis of molecular functions through synthesis has become an inherent requirement of synthetic disciplines. The low predictability of organic chemical reactions is the bottleneck of organic synthesis. The lack of reactivity of organic molecules in the bonding between molecules leads to the separation and purification of products, which is the main reason for restricting high-throughput and modular synthesis modes. These problems are determined by the relatively thermodynamically unstable organic compounds themselves, the structure and the diversity of functional groups. In response to these problems, K. Barry Sharpless proposed a modular click chemistry concept in 1999, based on the laws of chemical reactivity itself. The CuAAC reaction (the monovalent copper-catalyzed cycloaddition reaction of terminal alkyne and azide, 2002) and the SuFEx reaction (hexavalent sulfur-fluorine exchange, 2014) discovered by his group are the two most representative reactions in the field. . Compared to other chemical reactions, click chemical reactions are highly predictable in complex environments and have been widely used in many interdisciplinary fields. Extremely high reaction trends and near-orthogonal reactivity are prerequisites for the success of click chemistry; but it is also because of this, such reactions must use naturally available, high-energy reactive functional groups. The synthesis space of click chemistry is greatly limited by the availability of azide compounds and terminal acetylene compounds compared to the most widely used coupling reactions in medicinal chemistry. This has also led to the current greatest use of this method for linking molecules under complex conditions rather than the diversity of synthesis in the traditional sense. The unique synthetic, highly predictable reactivity is not fully realized.
In the process of finding a new SuFEx reaction block, Dong Jiajia's research team unexpectedly discovered a safe and efficient method for synthesizing rare sulfur (VI) fluorine-based inorganic compound FSO2N3 (fluorosulfonyl azide). Interestingly, this compound does not perform the SuFEx reaction as planned, but instead exhibits an unusually high diazo transfer reactivity for the primary amine functional group: the traditional diazo transfer reaction is an equilibrium process, and the reaction conditions generally require metal catalysis. And excess transfer reagent, so the separation and purification process is required; while FSO2N3 can carry out the diazo transfer reaction, the primary amine functional group can be rapidly and orthogonally obtained under mathematical conditions (1:1) under mild conditions. Converted to the corresponding azide. The reaction is equally effective for alkyl, aryl, sulfonyl substituted primary amines; they validate the universality of the reaction for substrates using a range of complex natural products and drug molecules. During the research, they further found that the FSO2NH2 molecule formed by FSO2N3 after diazo transfer was rapidly hydrolyzed into F- and NH2SO3- under two-phase conditions. Although the mechanism is still unclear, this unexpected process is likely to be one of the reasons for the balanced transfer of the reaction.
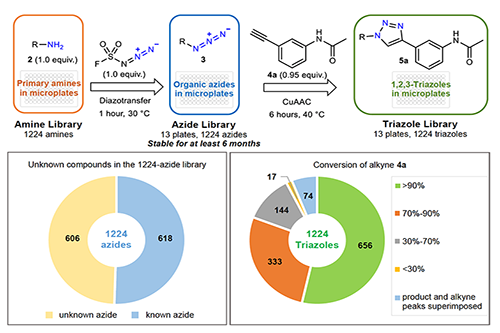
The primary amine is undoubtedly the most diverse functional group in organic chemistry with the highest block availability. Based on this newly discovered reaction, they synthesized the corresponding azide block library (1224) directly from a 96-well plate from a large number of available primary amine functional molecular blocks. The compound library does not need to be separated. Purification can be carried out by further performing a cycloaddition reaction (single-handed operation) with any given terminal alkyne compound in a 96-well plate, thereby directly performing functional screening. Under the premise of achieving great diversity of blocks and high predictability of links, a highly predictable high-throughput synthesis model was established (Fig. 2, modular click-click compound library method). They called the diazo transfer reaction of this newly discovered FSO2N3 with a primary amine compound a third click chemical reaction, and named the process a modular click-click compound library method. In addition to being compatible with known combinatorial chemistry methods such as DNA-encoding compound library methods, this method has several salient features compared to the previous combinatorial compound library method: the compound library is not displayed as a mixture and can be directly applied. Phenotypic screening for biological functions; due to the orthogonality of the click reaction and the biocompatibility of the reaction conditions, the range of precursor molecules that can be engineered is extremely large; the process normalizes the substrate reaction conditions for different structures, and does not require The separation and purification process directly performs the screening of functional molecules; the process is simple, and the target molecules after confirmation can be scaled up and above in a very short time and rapidly advanced. These features make this method a low-cost reproduction.
With the support of the Shanghai Organic Institute, the team has now pushed the number of primary amine blocks to more than 5,000. Dong Jiajia believes: "Based on this modular synthesis method, it is feasible to carry out more than 10,000 transformations of a given drug small molecule or macromolecular building block in a short time. The improvement of synthetic efficiency will lead to the discovery of drug lead molecules. Direct role." The work was published in Nature, entitled "Modular Click Compound Library Based on a Diazo Transfer Agent for Functional Screening." The team is using this method to actively and extensively cooperate with the Chinese Academy of Sciences academician Lin Guoqiang and Shanghai University of Traditional Chinese Medicine professors Zhang Jiange, Ke Xisong team, and Scripps Research Institute, hoping to first develop the drug in cancer, tuberculosis and other diseases. The practicability of the method was tested and a series of interesting experimental results have been obtained.
The research team applied for patent protection for reagents, reactions, and compound library methods required to implement the method before the article was published. The research work was supported by the Shanghai Institute of Organic Science, the National Natural Science Foundation of China, the Strategic Science and Technology Special Project of the Chinese Academy of Sciences (Class B), the Frontier Science Key Research Project of the Chinese Academy of Sciences, and the Shanghai Science and Technology Commission.
For this work, Nature and C&EN reported and commented on the first time.